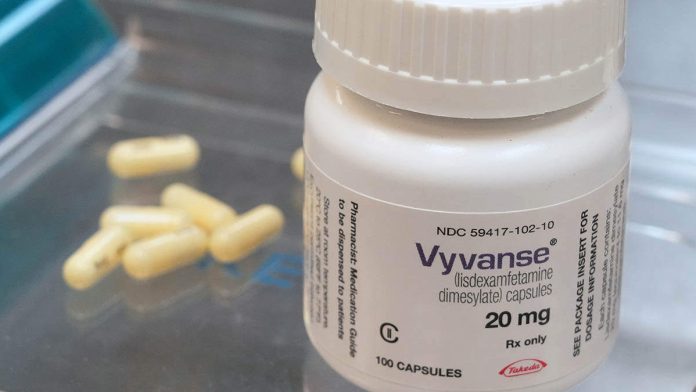
The U.S. Drug Enforcement Administration has increased the production limit for Takeda Pharmaceutical’s ADHD drug Vyvanse and its generic versions by about 24% to address the ongoing shortage in the United States. The raised production limit follows the Food and Drug Administration’s request in July, according to a DEA notice.
ADHD drugs have been in short supply for years. The FDA warned of a shortage of Israel-based drugmaker Teva Pharmaceutical Industries’ Adderall in October 2022, troubled by manufacturing delays. This led to a spike in demand and subsequent shortage of Takeda’s Vyvanse.
Vyvanse, also known as lisdexamfetamine, is classified by the DEA as a schedule II controlled substance, which is applied to drugs considered to have a high likelihood of being abused, and additional prescribing safeguards are put in place. The production limit for lisdexamfetamine was increased by 6,236 kilograms (kg), which includes 1,558 kg to address increased domestic demand and 4,678 kg for increased foreign demand for finished dosage medications, according to the DEA.
“These adjustments are necessary to ensure that the United States has an adequate and uninterrupted supply of lisdexamfetamine to meet legitimate patient needs both domestically and globally,” the DEA said.
The FDA approved generic versions of Vyvanse from 11 drugmakers, including U.S.-based drugmakers Mallinckrodt and Viatris, UK-based Hikma Pharmaceuticals, and Indian drugmaker Sun Pharmaceutical Industries, last year after Takeda lost exclusivity over the drug.
Recent Comments